-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
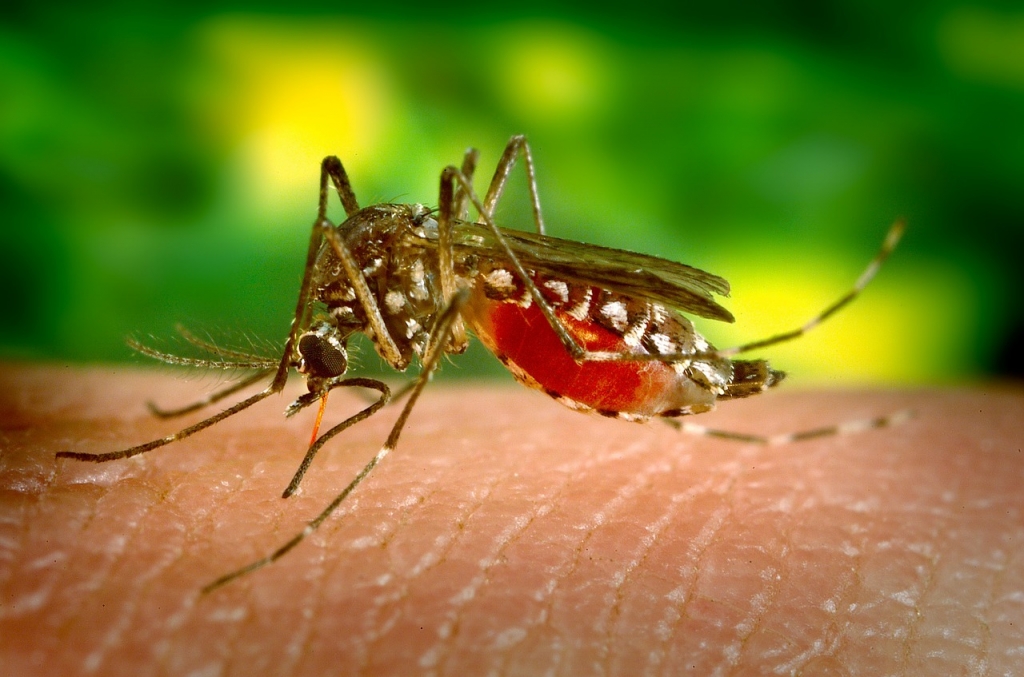
Sanofi’s dengue vaccine, Dengvaxia gets Brazilian approval
Brazil’s ministry of health was forced to approve vaccination with Dengvaxia when dengue cases rose to a phenomenal 1.5 million, as against the 555,400 cases recorded for 2014.
Advertisement
Olivier Charmeil, President and CEO of Sanofi Pasteur, pegged this approval as a major milestone in dengue prevention and public health and said that the approval is yet another evidence of Sanofi Pasteur’s long-standing commitment to introduce this innovative new vaccine first in countries where dengue is a major public health threat.
It was found that Dengvaxia could immunise two-thirds of people aged nine years and older, rising to 93 per cent for the more severe form of the disease, dengue haemorrhagic fever.
The vaccine will be approved for people ages 9 to 45 living in endemic areas, following research findings showing it proved most effective in that specific age group. On Monday, Brazil’s ANVISA approved Sanofi Dengvaxia dengue vaccine in hopes that it will help efforts to put an end to the ravaging dengue outbreak. Dengue is among the serious health concern tropical and subtropical countries in Latin America and Asia.
It was once considered a disease of the tropics, endemic in only nine countries. Most recently, the Philippines and Mexico faced similar dengue outbreaks.
Typical dengue fever symptoms include sever joint and muscle pain, skin rashes, headaches, nose bleeds and stomach pains.
Besides Brazil, Dengvaxia is also registered in Mexico and in The Philippines.
Sanofi Pasteur, the vaccines division of Sanofi, has been granted marketing approval for Dengvaxia® in the Philippines, making it the first vaccine to be licensed for the prevention of dengue in Asia.
The vaccine is a potential “blockbuster” drug for the company, which estimates it could generate more than $1 billion a year in revenue.
Dengvaxia is manufactured by French pharmaceutical giant Sanofi. Over 40,000 volunteers participated in the Sanofi Pasteur dengue vaccine clinical study programme (phase I, II and III), of whom, 29,000 volunteers received the vaccine.
Advertisement
Due to Brazil having a large number of rivers and lakes, the mosquito population is much higher than other countries. The agency and the company didn’t reveal when the vaccine, with the brand name Dengvaxia, will become accessible in Brazil. Its main task is to aid in preventing the disease that is caused by four dengue virus types namely the DEN-1, DEN-2, DEN-3, and DEN-4. First doses of the vaccine have been produced at the dedicated production site in France with planned full-scale production capacity of 100 million vaccine doses annually.