-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
Sarepta Wins Controversial FDA Approval for First DMD Drug
The US Food and Drug Administration has given an accelerated approval to the first drug of Sarepta Therapeutics Inc (NASDAQ:SRPT) that helps slow down further advancement in the disease, Duchenne muscular dystrophy. Exondys 51 is indicated for the treatment of DMD patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping, which is found in approximately 13% of this population.
Advertisement
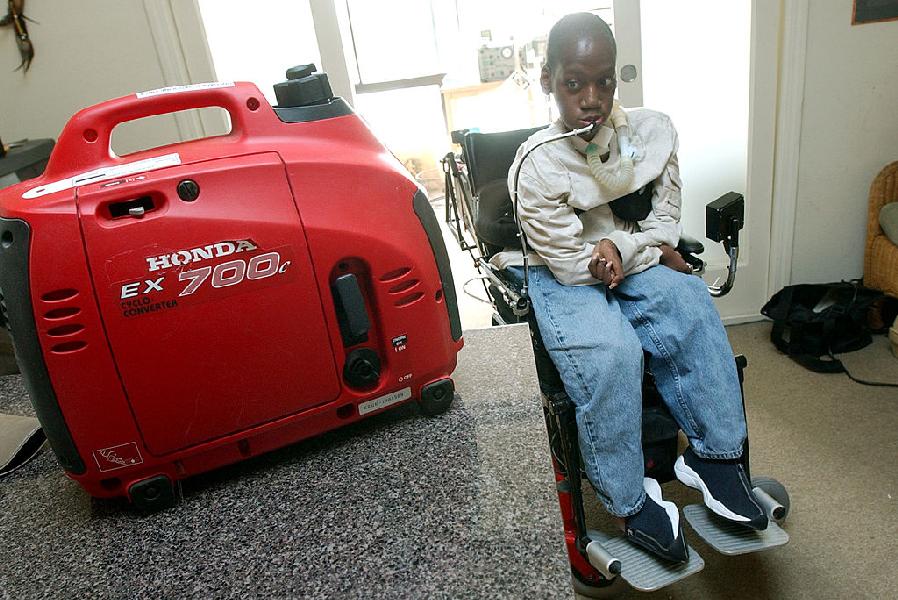
Her son Connor is autistic, but he is also living with Duchenne muscular dystrophy, a degenerative muscle-wasting disease. The drug acts on a protein called dystrophin, which plays a role in the growth of muscle fibers.
People with DMD typically die in their 20s or 30s from the progressive disorder, which affects about one of every 3,600 males worldwide.
As the disease and symptoms progress, many patients face heart and respiratory conditions that can significantly shorten lifespan.
Ultimately, the new injection, known as Exondys 51, was approved under FDA’s accelerated approval program, reserved for drugs to treat serious or life-threatening diseases, and where there is a lack of available therapy.
Findings from the study suggest that the drug can increase dystrophin production, and could potentially provide benefits for patients.
The FDA cleared Sarepta Therapeutics’ Exondys 51 for a rare form of Duchenne muscular dystrophy, a deadly inherited disease that affects boys.
The analyst said in another report this morning that the commercial launch of eteplirsen (Exondys 51) is “imminent, as the drug maker’s management said on their conference call that they’ve been prepared to launch it for six months”.
The FDA cautioned, however, that eteplirsen has not yet proven a real clinical benefit, and that Sarepta has to conduct an additional trial to show that benefit exists. Eteplirsen is created to enable RNA to skip over the part of the DNA with a problematic mutation, enabling a functional (though shorter) dystrophin protein to be produced.
“If postmarketing clinical trials fail to verify clinical benefit or are not conducted with due diligence, we may, following a hearing in accordance with 21 CFR 314.530, withdraw this approval”.
The drug’s most common side effects include balance disorder and vomiting. With the help of its supporters, MDA plans to double its research spend targeting treatments and clinical trials by the year 2020. She values the priority review voucher received by the company at $5 per share. Not all agreed, however, and the FDA’s advisory committee (which influences but does not mandate final decisions) voted 7-6 that the evidence just wasn’t there. The companys stock also got a bounce last week after the FDA confirmed a staff member who had been critical of the drug, Dr. Ronald Farkas, had left the agency for another job. For these boys, who had previously faced certain death and no treatment options, any improvement in their condition was to be heralded, despite limited clincial data.
Advertisement
Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, posted a 10-page letter to Cambridge, Massachusetts-based Sarepta, affirming the regulatory agency’s review of the application, and approving it for use as recommended in an agreed-upon labeling text.