-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
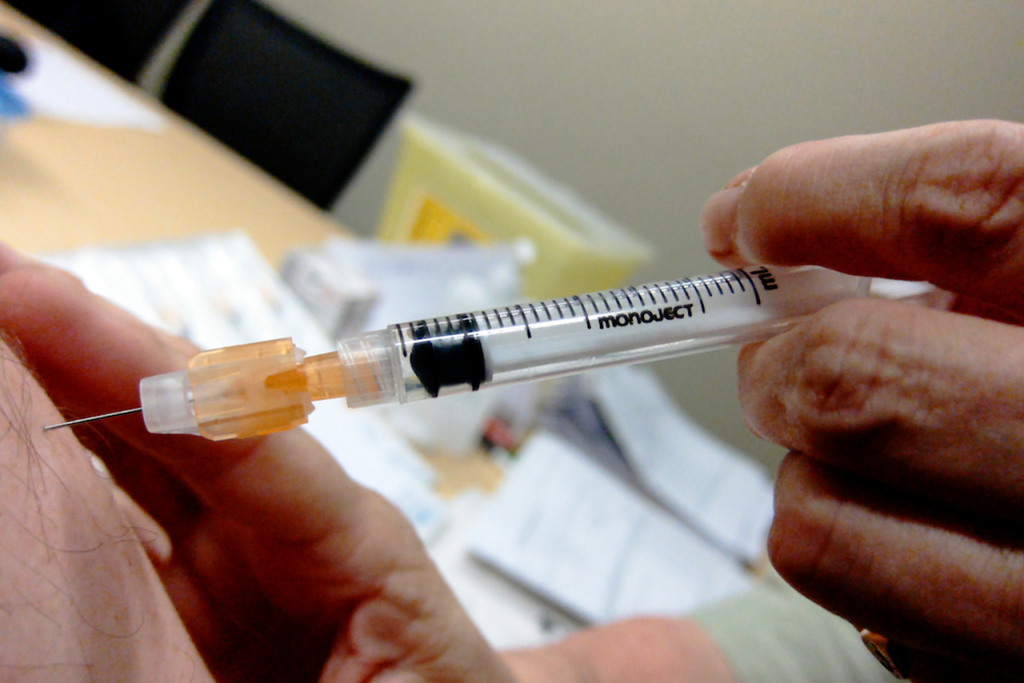
Second Zika vaccine trial gets under way in US
The early-stage study will take place at three study sites in the US with at least 80 healthy volunteers ranging in age from 18 to 35.
Advertisement
The approach being used to the NIAID Zika virus investigational DNA vaccine is similar to that used for another investigational vaccine developed by NIAID for West Nile virus. Should the Phase II trial start on time in January, it will not be until the following year that researchers get the results.
It can not infect people with Zika, but is created to make the body mount an immune response to Zika. There is now no vaccine to prevent Zika. Last week, Florida health officials identified four cases of the Zika virus that were likely transmitted by mosquitoes, which will make efforts to combat the illness even more hard.
A push for $1.9 billion by President Barack Obama to fight the Zika virus has remained stalled in Congress, which is now out of session for the August recess. Zika virus infection during pregnancy can cause a serious birth defect called microcephaly, as well as other severe fetal defects of the brain and other organs.
“It is our hope that this study will yield the encouraging results necessary to move to a Phase 2 clinical trial”, Fauci said.
Dr Ed Wright, senior lecturer and virologist at the University of Westminster, said: “All of the vaccines now under development are many years away from being licensed and available for widespread public use”.
Meanwhile, South Florida is now reporting 15 cases of local Zika transmission.
“A safe and effective vaccine to prevent Zika virus infection and the devastating birth defects it causes is a public health imperative”, NIAID Director Anthony Fauci said in the release.
USA health officials had previously said they hoped to start testing their vaccine in September, but they were able to expedite its development, showing how quickly scientists are trying to come up with tools to combat the virus. In some ways, they are easier to develop and could come with fewer risks than vaccines made from inactivated forms of the virus, Fauci said.
Even if the Zika vaccine isn’t ready in time to prevent infections in Brazil, the country hardest hit by Zika, the vaccine could help prevent cases elsewhere in the world, said William Schaffner, a professor at the Vanderbilt University School of Medicine.
Numerous reported Zika cases in the continental USA involved people who have traveled to Zika-infected countries.
Advertisement
According to the Centers for Disease Control and Prevention, it is “likely” that an individual is protected from future infections once they have been infected by the virus.