-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
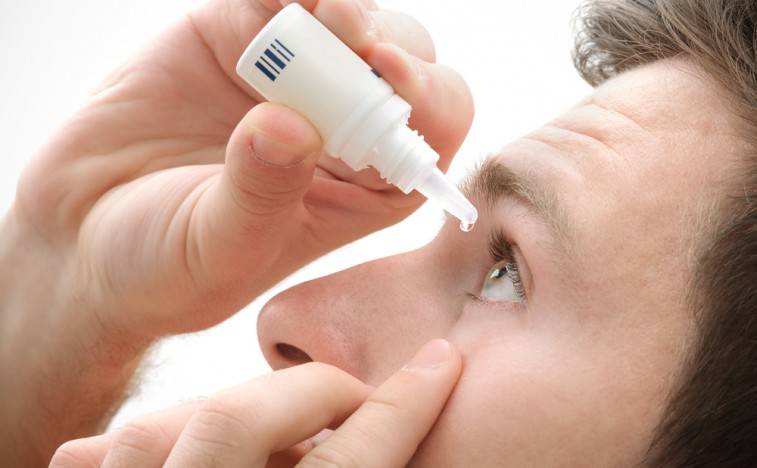
Shire Gains Key FDA Approval in Dry Eye Treatment
Shire ($SHPG) has been given a key USA approval for its new eye disease drug lifitegrast almost a year after it was rejected by the FDA, and can now look to make good on its expected blockbuster sales.
Advertisement
Many older Americans experience dry eye disease, which the FDA says affects about 5 percent of adults in their 30s, and up to 15 percent of people over the age of 65.
Shire’s medication had been dismisses at first by the Food Department Authorities in October, then Shire was requested an extra clinical concentrate, yet the organization had stayed certain that it could fulfil the US controller.
As Shire’s first FDA-approved medicine in ophthalmics, this significant milestone advances our goal of becoming the global leader in this category, where there are unmet patient needs.
Analysts have estimated the drug could generate more than $1 billion sales a year.
The most common side effects of Xiidra include eye irritation, discomfort or blurred vision when the drops are applied to the eyes, and an unusual taste sensation (dysgeusia).
Xiidra is also indicated for treatment of both signs and symptoms of dry eye disease, whereas Restasis was approved only on a demonstrated improvement in objective signs of dry eye. These drugs can not, however, help patients with dry eye disease.
Analysts at Shore Capital said the only competition Shire will face in this market is from Allergan’s Restasis.
The Dublin based drugmaker has always been a major securities exchange champ taking after England’s choice to abscond the European Union, profiting from the quality of the USA dollar against sterling and speculator interest for protective divisions like pharmaceuticals.
Advertisement
“Right now large-scale M&A is not on the cards for us, with the integration of Baxalta and the focus on delivering on opportunities like the launch of Xiidra”, he said.