-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
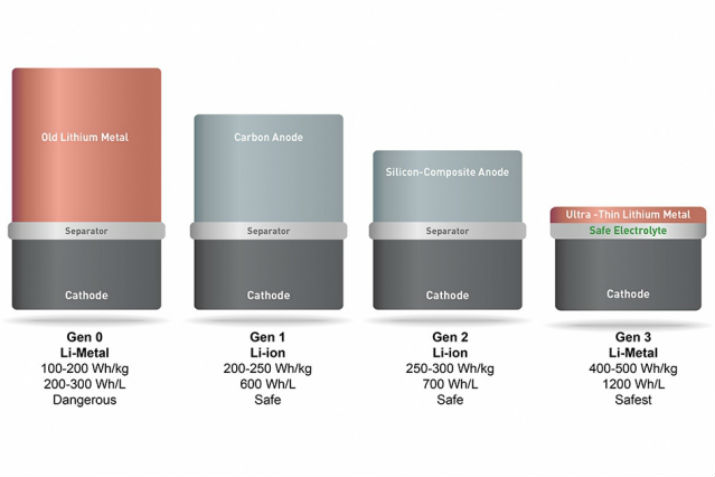
Solid lithium garnet batteries improve safety
Founded in 2012 by MIT alumnus and former postdoc Qichao Hu ’07, SolidEnergy Systems has developed an “anode-free” lithium metal battery with several material advances that make it twice as energy-dense, yet just as safe and long-lasting as the lithium ion batteries used in smartphones, electric cars, wearables, drones, and other devices.
Advertisement
ARENA CEO Ivor Frischknecht told the launch gathering that while many Australians were hanging back waiting for battery storage prices to fall, this would not be the case for much longer, with costs predicted to fall by 60 per cent by 2020.
Lithium metal, for one, reacts poorly with the battery’s electrolyte – a liquid that conducts ions between the cathode (positive electrode) and the anode (negative electrode) – and forms compounds that increase resistance in the battery and reduce cycle life.
Now, the ETH team has developed an effective method to achieve this – constructing a battery with a layer of highly conductive lithium garnet, which works as a solid electrolyte between the two electrodes.
To tackle these drawbacks, Hu created a liquid and solid hybrid electrolyte solution. Lithium garnet is one of the materials with the highest known conductivity for lithium ions.
According to MIT, researchers have long eyed the potential for lithium metal batteries, but haven’t been able to overcome negative effects of the material reacting to the battery’s electrolyte.
Measures taken to make the batteries safer come at the cost of the battery’s energy performance, such as switching out the liquid electrolyte with a poorly conductive solid polymer electrolyte that must be heated at high temperatures to work, or with an inorganic electrolyte that is hard to scale up.
But there was still a major setback: The battery only worked at 80 degrees Celsius or higher. This creates a larger contact area between the electrode and electrolytes, and means that the battery can be charged faster.
SolidEnergy Systems is now looking to commercialise its product, starting by bringing the battery into the drone market this coming November. The work was reported in the journal Advanced Energy Materials.
SolidEnergy debuted the first-ever working prototype of a rechargeable lithium metal smartphone battery with double energy capacity in October 2015, and earned more than $12 million from investors. By coupling battery power plants with industrial facilities, you could use the waste heat to operate the storage power plant at optimal temperatures, ‘ he said.
Researchers have tried to make rechargeable lithium metal batteries for decades, with no success, Hu said. However, there are few studies such as this one, in which the scientists assembled an entire solid-state battery – using methods also used in industrial production – and tested it. “Thus, we have shown that it is possible to build whole batteries based on lithium garnet”, said Prof Rupp. Thanks to this solid electrolyte one cannot only operate batteries at higher temperatures, but also build thin-film batteries, that can even be directly placed on silicon chips.
Advertisement
Researchers at Switzerland’s ETH Zurich have come up with a battery design they say addresses this problem. The immediate next step is to optimise the battery, with a particular focus on further increasing the conductivity of the electrode-electrolyte interface.