-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
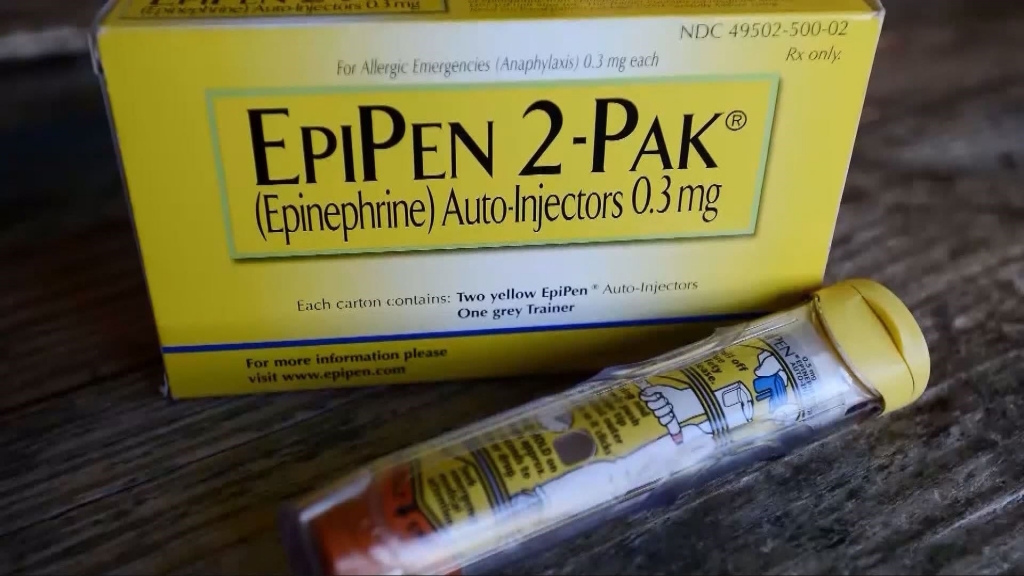
State senator introduces resolution to condemn EpiPen price hikes
In its latest move to quell outrage over its price increases, the maker of the EpiPen has resorted to an unusual tactic – introducing a generic version of its own product. Now that product and a couple rival brand-name ones could hit the US market in mid- to late 2017.
Advertisement
U.S. Senator Joe Manchin said he believes many of his Senate colleagues will be interested in hearing the ins and outs that have led to the price point controversy over Mylan Pharmaceutical’s EpiPen.
Mylan has repeatedly pointed to high-deductible health plans, which leave patients with more out-of-pocket costs, as the main reason that patients are suddenly noticing higher prices for EpiPens. The product will be available as a two-pack carton, with versions containing 0.15 and 0.30 mg of epinephrine, which is used to quickly treat the severe allergic reaction known as anaphylaxis.
This way, Mylan can keep its original list price up on the EpiPen while keeping users who might be deterred by its price from going to a competing emergency-epinephrine device.
Some pharmacists argue that the cheaper generic drug really isn’t a price adjustment at all for patients.
Anyone with a prescription can buy the device that administers the medication.an EpiPen, but for a price of more than $600. It had cost $100 in 2009. It will continue to market and distribute a branded EpiPen. The difference between the revenue and the list price comes from discounts and fees given to pharmacy benefit managers, insurers and distributors.
There is now little competition for EpiPen, with the only rival product being Adrenaclick, which carries a list price of $461.
At least two companies are seeking approval to sell a rival brand or generic version of EpiPen in the United States, but none are likely to be available until later next year. The site crunched the numbers and determined that selling a generic EpiPen pack might net Mylan $26 more than a sale of its branded product.
Asked about the letter, Mylan spokeswoman Lauren Kashtan said in an email statement: “We have acknowledged receipt of letters from congressional offices and intend to respond to them”.
Sen. Lisa Murkowski has joined a growing group of USA lawmakers calling for an investigation into a pharmaceutical company’s controversial decision to sharply increase the price of EpiPens.
Advertisement
It has also asked company officials to appear for a private briefing by September 6 – just six days before the paperwork is due.