-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
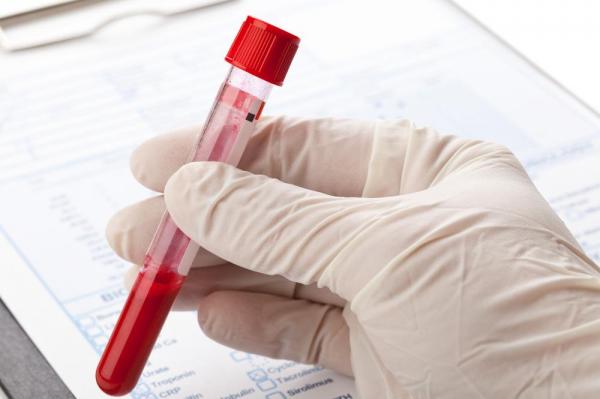
The FDA thinks Pathway Genomics’ cancer blood test could be harmful
LDTs now do not need to be approved by FDA, though this letter could raise further questions about FDA’s plan to regulate LDTs as medical devices.
Advertisement
It says there is no evidence at all that it works, and warns that the “high risk” test may actually harm public health.
Pathway Genomics posted a white paper on its website about the analysis of 96 “hotspot mutations”, but the FDA was unimpressed, writing, “It is unclear how the literature that you cited, addressing the presence of circulating tumor DNA (ctDNA) in already-diagnosed patients, is adequate to support the expansive claims of screening for early cancer detection using ctDNA in undiagnosed patients for up to 10 different cancers with the CancerIntercept Detect”.
The FDA said that Pathway’s testing kit counts as a medical device, and that the San Diego-based company hasn’t applied for regulatory clearance.
It is marketed as an early warning system for people who are at high risk of developing cancer but don’t have any symptoms.
The agency says it has not found any published evidence that the diagnostic, or a similar test, has been validated to screen high-risk subjects for early-stage cancer.
Doctors have been enthusiastic about the potential of so-called liquid biopsies as a low-priced, non-invasive alternative to traditional tissue samples.
From the company’s perspective, Pathway, which is backed by IBM and the venture capital company Founders Fund, is adamant that it assures physician involvement in ordering and reviewing CancerIntercept testing, as well as follow-up. Instead, Pathway said it’s governed by a U.S. Centers for Medicare and Medicaid Services program that ensures labs can perform accurate tests on patients.
In a letter to Pathway Genomics, the FDA has expressed interest in discussing the company’s validation strategy as well as evaluating data on clinical sensitivity and specificity of the CancerIntercept Detect test.
“We believe that CancerIntercept Detect is a laboratory developed test and, as a CLIA and CAP certified clinical laboratory, we are offering at such”, Pathway Genomics wrote.
This is not the first time that the company has received push-back from the FDA.
Advertisement
Jim Plante a letter in 2010 about the lack of FDA clearance for its Genetic Health Report, a saliva collection kit that purported to tell consumers their risk for certain diseases and likelihood of responding to certain medications. The FDA said 23andMe didn’t have agency clearance.