-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
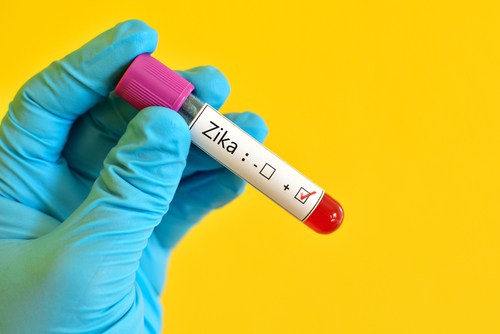
UMD School of Medicine to host Zika vaccine clinical trial
At the moment, there are no DNA vaccines approved for use on humans in the US, but apparently the Zika vaccine is similar to one developed for West Nile virus, which is considered to be safe.
Advertisement
Separately, Pentagon officials said Wednesday that more than 30 active-duty US service members – including a pregnant woman – have contracted the Zika virus in countries where the disease has been identified.
With more than 50 countries and territories battling active Zika outbreaks, the need for a vaccine is clear.
The trial will let scientists and researchers know whether the vaccine is safe for usage on humans and the effect that it has on immune system response will also be measured.
Dr. Anthony S. Fauci, director of NIAID, said creating a safe and effective Zika virus vaccine is a public health priority. At least 80 healthy volunteers ages 18-35 years at three study sites in the United States, including the NIH Clinical Center in Bethesda, Maryland, are expected to participate in the trial.
“These data support the rapid clinical development of [Zika virus] vaccines for humans”, the study authors concluded. If results show a favorable safety profile and immune response, NIAID plans to initiate a Phase 2 trial in Zika-endemic countries in early 2017. Babies born with microcephaly have abnormally small heads, leading to mental and physical disability.
Worldwide, most Zika cases have occurred in Latin America.
The threat of contracting Zika has led for some athletes to skip the Summer Olympics in Rio de Janeiro, Brazil. This news comes after the United States Department of Defense (DoD) confirmed more than 30 cases of Zika among U.S. troops. As of Tuesday, Florida health officials said that 15 people had been infected. Among other recommendations, the CDC urges pregnant women not to travel to Wynwood, a neighborhood just north of the downtown Miami, where the virus seems to be spreading.
On 1 February, the World Health Organisation declared Zika a public health emergency of worldwide concern.
Last month started testing another experimental vaccine developed by the laboratory Inovio Pharmaceuticals and the South Korean biotech Genone Life Sciences.
The Zika virus has been particularly concerning to federal health officials because it is known to cause birth defects.
In the monkey study, one vaccine followed the traditional approach, using a dead Zika virus to train the body for fighting off infection. In comparison to other viruses, this shows that the Zika virus seems to potentially be relatively easy to vaccinate against.
The experimental vaccine was developed earlier this year by experts at the National Institute of Allergy and Infectious Diseases’s (NIAID) Vaccine Research Center. “The results of animal experiments were very encouraging”.
Human trials are set to begin, according to Barouch.
But Gilbert, who was not involved in the research, advised caution in proceeding with testing the vaccines in humans.
Advertisement
Beth Israel researchers and the Walter Reed Army Institute of Research previously tested two of those vaccines on mice.