-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
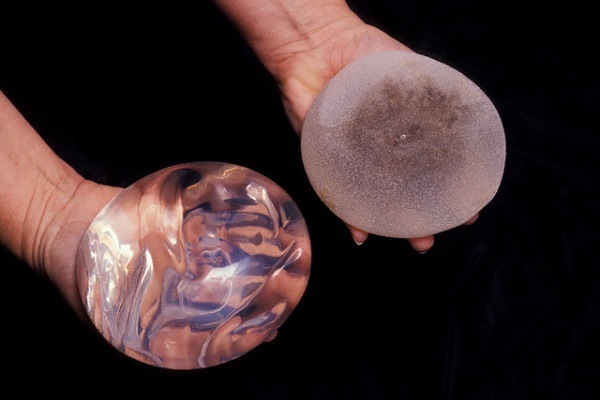
United Kingdom regulator suspends sale of Silimed’s silicone implants
Device Technologies, the Australian and New Zealand distributor of Silimed silicone implants, including breast implants, has voluntarily suspended the distribution of these products and has accordingly advised surgeons not to use these products until further notice.
Advertisement
A German authority appointed to monitor Silimed “has recently carried out an inspection of the manufacturing plant in Brazil and established that the surfaces of some devices were contaminated with particles”, it said in a statement.
The MHRA statement said however that “for the moment there has been no indication that these issues would pose a threat to the implanted person’s safety”. Some of their products include breast, pectoral and calf implants, as well as gastric bands and gastric balloons. There’s still no word on whether the devices pose a threat to individuals with the implants.
Medsafe has started an “urgent investigation” into all Silimed silicon breast implants.
‘In the meantime, we would recommend that people with these implants who have questions should seek advice from their surgeon or clinic’.
The MHRA monitors the safety of all medical devices.
The announcement follows the PIP implant scandal which saw the NHS announce in 2012 that it would remove all PIP implants for the approximate 47,000 British women believed to be given the faulty non-medical grade silicone.
The implants were at least twice as likely to rupture than other brands.
“We don’t know any more at this stage, other than the manufacturing licence and Australian sales have been suspended”, said ASPS spokesman Dr Rod Cooter. The CE mark guarantees that a manufacturer has checked that its products meet European Union safety, health or environmental requirements.
The authority is working with the Irish supplier to identify if products imported into Ireland are affected by these issues, and is also working with Silimed and the testing body based in Germany to establish the level of risk involved.
Advertisement
According to MHRA, its investigation with other European regulators is in progress and it recommends that no device of the company should be implanted until further recommendation.